
Be confident of medical device market approval
Be confident of medical device market approval
Based on the product classification, the manufacturer must apply for an applicable conformity assessment procedure.
The application forms requesting detailed information can be accessed here.
Step-by-step information for each of the conformity assessment procedures (using the relevant Annex) is highlighted below. The graphics also provide an overview of the procedures for different device classes and types, as well as relevant surveillance activities. Specific device types require additional assessments, which are listed in the tables below.
English and/or German are the only acceptable languages for the submission of documentation and any related correspondence.
The certification costs are based on hourly rates and take into account factors such as the size of company, number of sites, and number and complexity of devices, etc.
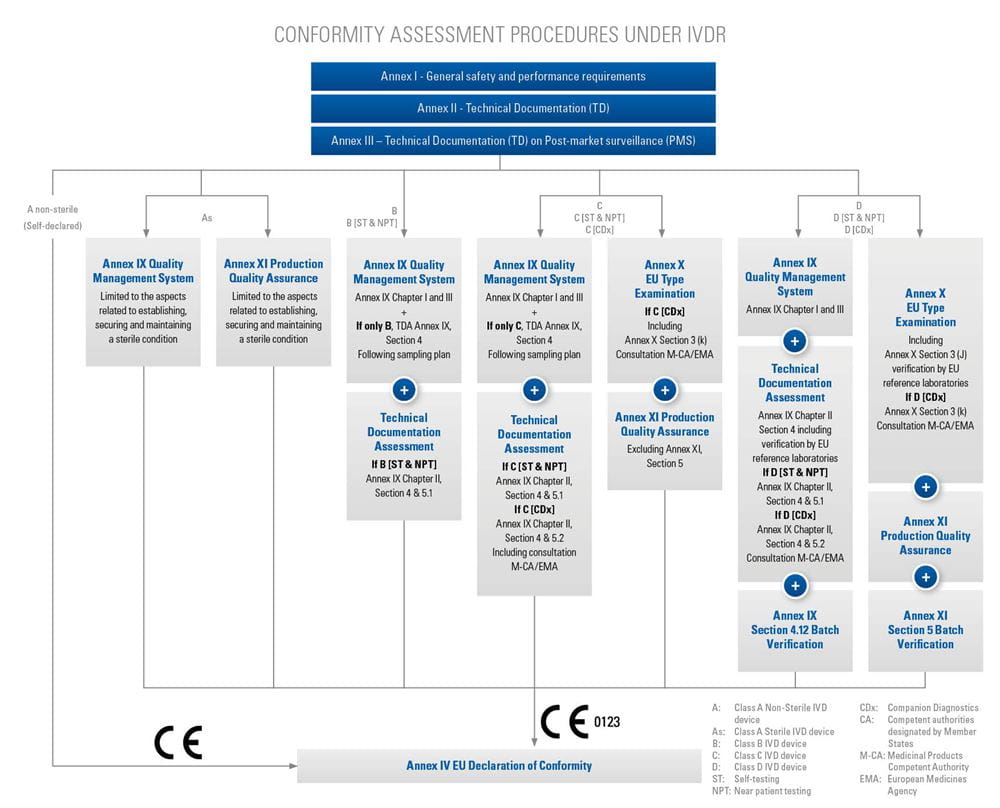
+Refer to the Nando website for the applicable products and procedures/annexes (see Notification)
CONFORMITY ASSESSMENT BASED ON A QUALITY MANAGEMENT SYSTEM AND ON THE ASSESSMENT OF TECHNICAL DOCUMENTATION
Chapter I: Quality Management System (QMS)
1. Application Management
3. Technical Documentation (for Classes B, C on sampling basis, n/a for specific types of devices)
(Assessment of the technical documentation is performed before or during routine audits)
Chapter II: Assessment of Technical Documentation (additional for Classes D devices, incl. self-testing, near-patient-testing, Class C companion diagnostics; and Class B & C devices for self-testing, near-patient-testing)
2. Technical Documentation Assessment
3. Certification
4. Annex IX, 4.12: verification of each manufactured batch of Class D devices (Batch verification, incl. testing at EU Reference Laboratory), including reporting of the decision to the manufacturer
Annex X in addition to Annex IX for classes D and C including
Self-Testing, Near-Patient-Testing and CDx, if applicable
2. Assessment of Technical Documentation
Customer receives deficiency report (if applicable)
Customer addresses deficiencies
For Class D: verification of claimed performance by EU Reference Laboratory, including testing
Partial report on technical documentation assessment
3. Testing
4. Certification
5. Additionally required: conformity assessment according to Annex XI
CONFORMITY ASSESSMENT BASED ON PRODUCT QUALITY ASSURANCE
ANNEX XI for Class A-sterile without applying Annex X IVDR
ANNEX XI - in addition to Annex X for Classes D and C, including self-testing, near-patient-testing and CDx, if applicable
1. Application Management
2. Auditing (Production QMS)
3. Technical Documentation (not applicable in cases where Annex XI is in addition to Annex X as this has already been assessed) - only for Class A (sterile)
Please note: Assessment of the technical documentation is performed before or during the routine audits
4. Certification
5. Verification of each manufactured batch of Class D devices (Batch verification, incl. testing at EU Reference Laboratory), including reporting of the decision to the manufacturer
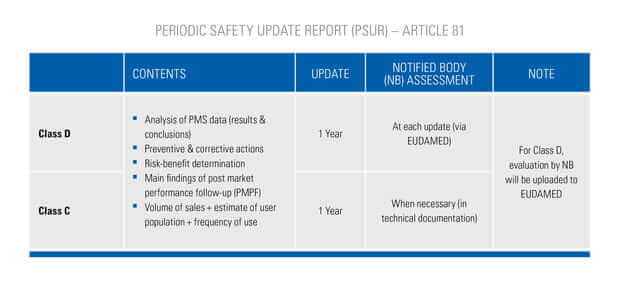
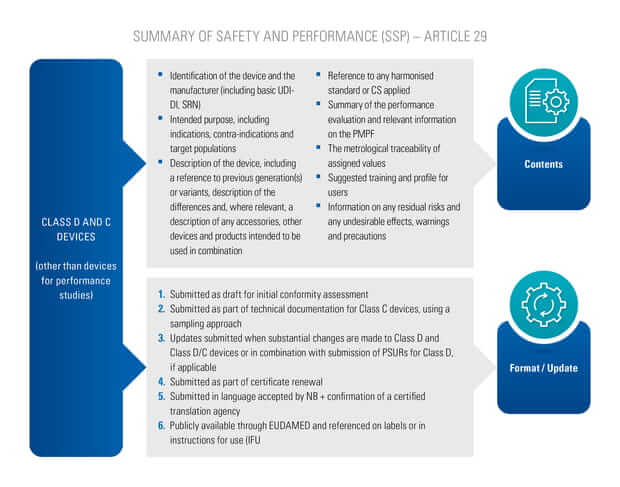
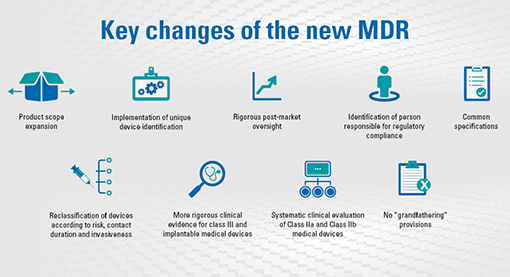
On May 5th 2017, the European commission has published a new regulation for medical devices.
Learn more



Site Selector
Global
Americas
Asia
Europe
Middle East and Africa