
将您的医疗设器械测试水平提升到全球标准
将您的医疗设器械测试水平提升到全球标准
植入物等医疗器械,如果处在磁共振成像(MRI)扫描仪环境中或附近,会因为电磁相互作用(如射频加热)给患者带来风险。医疗器械的MRI安全性测试是确保患者安全、植入物性能正常,且符合法规的必要条件。
我们有着最先进的实验室、技术高超的专业人员、全面的国际认可以及CB scheme资质,有助于您在医疗器械认证的同时获得市场认可。"
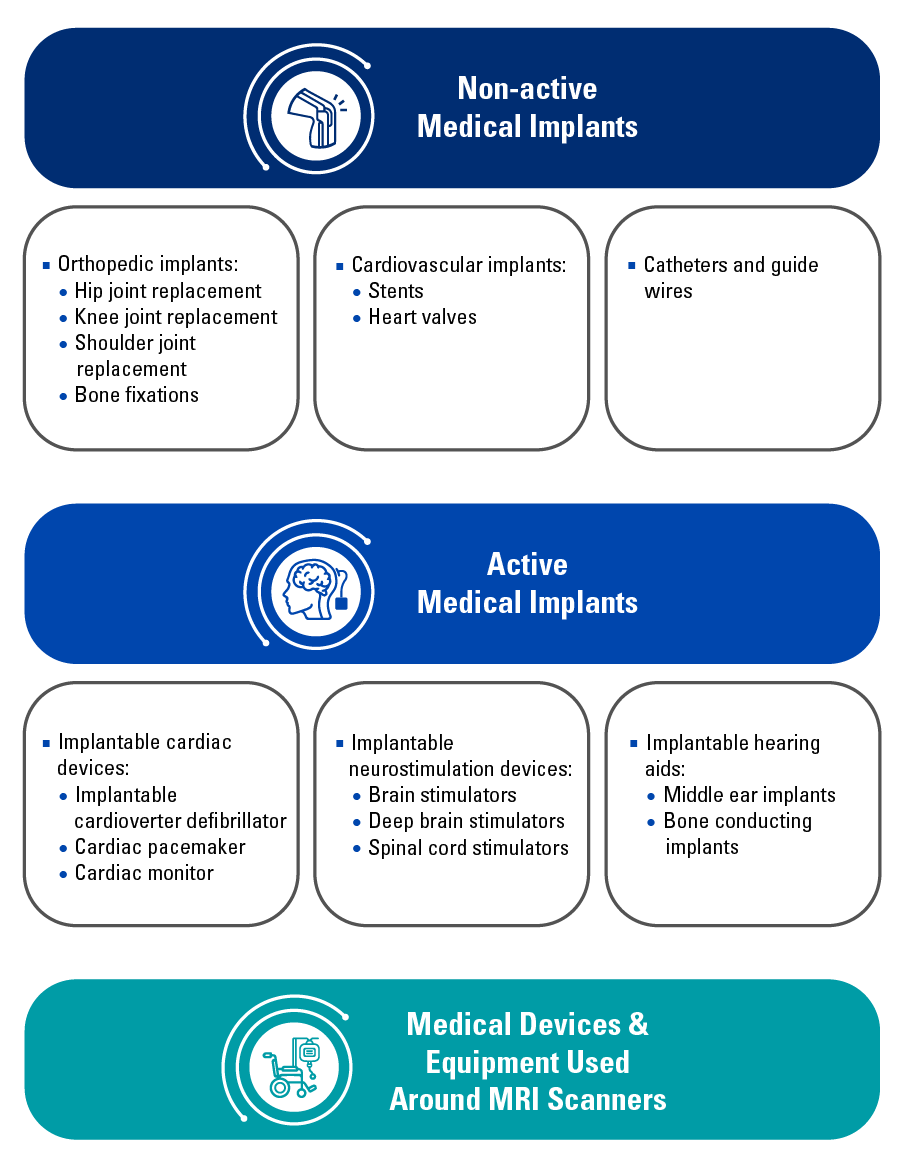
TÜV南德意志集团是各种医疗器测试服务的主要供应方之一。我们在明尼苏达州的实验室可以测试以下示例器械的MRI安全性:(这不是一份完整的清单,更多细节请联系medicaldevice@tuvsud.com)
TÜV南德意志集团提供全方位的服务和测试,以评估医疗器械在MRI环境下的安全性。
我们的服务概览:

If you are unable to find the service you require, contact us. Our laboratory offers a wide range of services to meet your needs.
WHY CHOOSE TÜV SÜD
Key market approvals from a single partner
Marketing advantage and a path to a competitive MR-Labeling
Active involvement in standards development and implementation
The MRI safety testing of a medical device is necessary to ensure patient safety, implant performance, and compliance with regulations. TÜV SÜD's state-of-the-art laboratories, highly skilled professionals, comprehensive international accreditations, and membership in the CB Scheme can facilitate your device's market approval in addition to its medical device certification.
Currently, the following types of medical devices should be tested for MRI safety:
The Food & Drug Administration (FDA) provides guidance to the industry with recommendations on testing to assess the safety and compatibility of medical devices in the Magnetic Resonance (MR) Environment. This guidance includes the recommended format for Magnetic Resonance Imaging (MRI) safety information in medical device labeling.
This guidance document provides recommendations on MRI safety and compatibility assessments and labeling information that should be included in its premarket submissions, including:
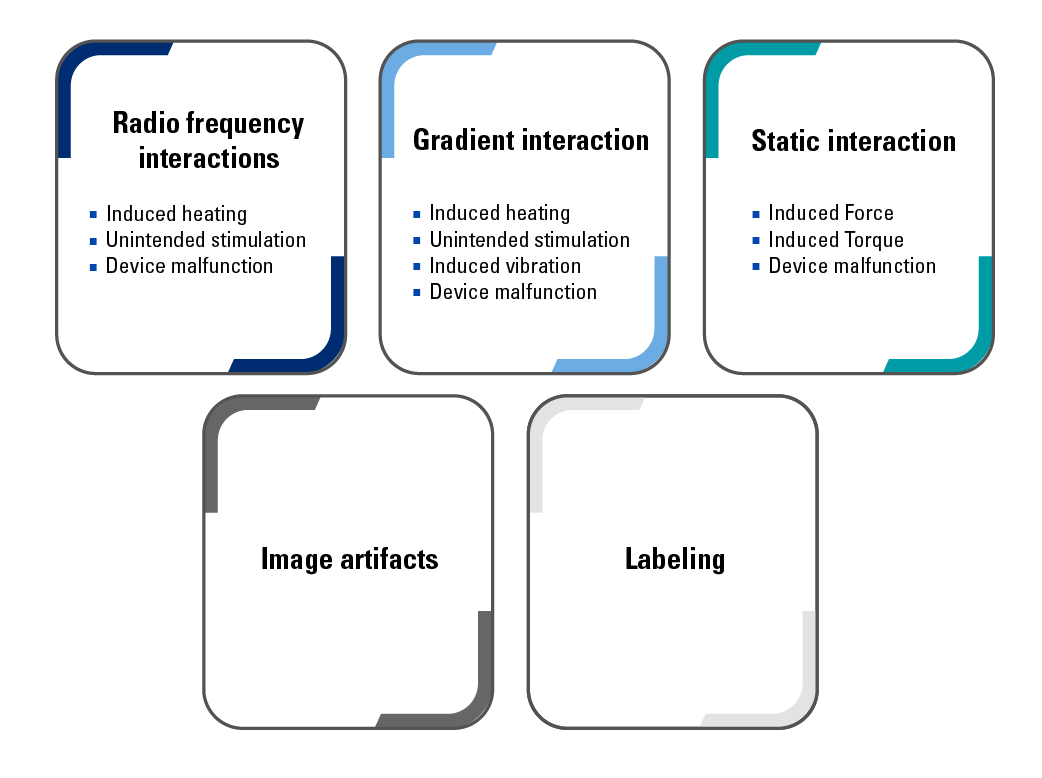
The EU Medical Device Regulations (MDR) require medical device manufacturers, especially implant manufacturers, to identify and reduce the risks associated with MRI scanners to ensure patient safety in the MRI environment. Interaction with the Electromagnetic fields of MRI scanners is considered a foreseeable risk, and manufacturers are required to label the devices appropriately. Any device containing metallic, magnetic, or conductive components must be evaluated and tested for RF-induced heating, image artifact, and magnetically induced displacement force and torque.
The MR environment has a variety of unique safety hazards, particularly for patients with implants, external devices, and accessory medical devices. The FDA has implemented a labeling scheme that categorizes whether a medical device or implant is unsafe, conditionally safe, or completely safe to be introduced to an MRI environment.
According to the FDA, here is the current breakdown of MRI Safety Labeling:
Because joint deterioration and corrective orthopedic and surgical procedures are typically monitored via magnetic resonance imaging, many non-active medical implants such as hip joint, knee joint, and shoulder joint replacements must undergo MRI safety testing to assess how these implants will interact and impact an MRI environment. Similarly, cardiovascular medical device implants such as heart valves, stents, catheters, and guidewires should also be thoroughly tested to ensure patient safety.
An AIMD or Active Implantable Medical Device is a device whose operation depends on an electrical energy or power source other than what is generated by the human body or gravity. AIMDs sometimes contain accessories such as lead wires, controllers, and battery packs to operate.
It is intended to be entirely or partially introduced to the human body via surgical or medical means. These devices are implanted to remain post-procedure for extended periods and are subject to more rigorous controls in both the pre-and post-market.
Electrically active devices such as AIMDs can be hazardous within the MRI environment because of the potential interaction with status magnetic fields, RF fields, and gradient fields present, which could cause device failure.
Site Selector
Global
Americas
Asia
Europe
Middle East and Africa