MEDICAL DEVICE APPROVALS IN SOUTH KOREAN MARKET
With a population of over 50 million people, South Korea is one of the world's leading economic powers. As the elderly population increases, the country is spending more on healthcare than other Asian countries, such as Hong Kong, Singapore, and Taiwan. South Korea has become increasingly attractive to medical device manufacturers worldwide, as more than half of the approved medical devices are being imported from overseas.
legal trends for medical devices in south korea
Manufacturers and suppliers intending to sell or import medical devices in South Korea must first obtain approval from the Ministry of Food and Drugs Safety (MFDS). Typically, both domestic and foreign manufacturers of imported medical devices are subject to GMP audit.
In South Korea, medical devices are categorised into four classes based on their level of risk, and procedures vary according to the class.
- Class I: Registration
- Class II: Certification
- Class III and Class IV: Approval from the MFDS
For medical devices classified as Class II, Class III, and Class IV, including those with new types of technology or new intended uses, the MFDS requires technical documents and clinical studies based on Safety and Efficacy Review (SER). For Class III and IV medical devices, the MFDS directly reviews the submitted files. For Class II medical devices, a third-party organisation approved by the MFDS conducts the review on the Ministry’s behalf. Regarding the functionality, safety, and efficacy of medical devices, it is mandatory to obtain test reports from MFDS-approved testing laboratories and submit them.
MEDICAL DEVICE GOOD MANUFACTURING PRACTICE (GMP) AUDIT
The Medical Device Good Manufacturing Practice (GMP) Audit is an assessment conducted to determine whether medical devices can be guaranteed consistently high-quality production, ensuring they are safe, effective, and suitable for their intended purpose.
TÜV SÜD Korea has been designated as a GMP organisation for medical devices and in vitro diagnostic medical devices by the MFDS in accordance with relevant regulations.

Scope
- Individuals intending to manufacture medical devices or obtain import approval/certifications/registration for medical devices
- Individuals intending to manufacture or import medical devices for clinical trials
- Medical device manufacturers or importers intending to obtain conformity assessment or conduct a re-certification audit
Types of Audits
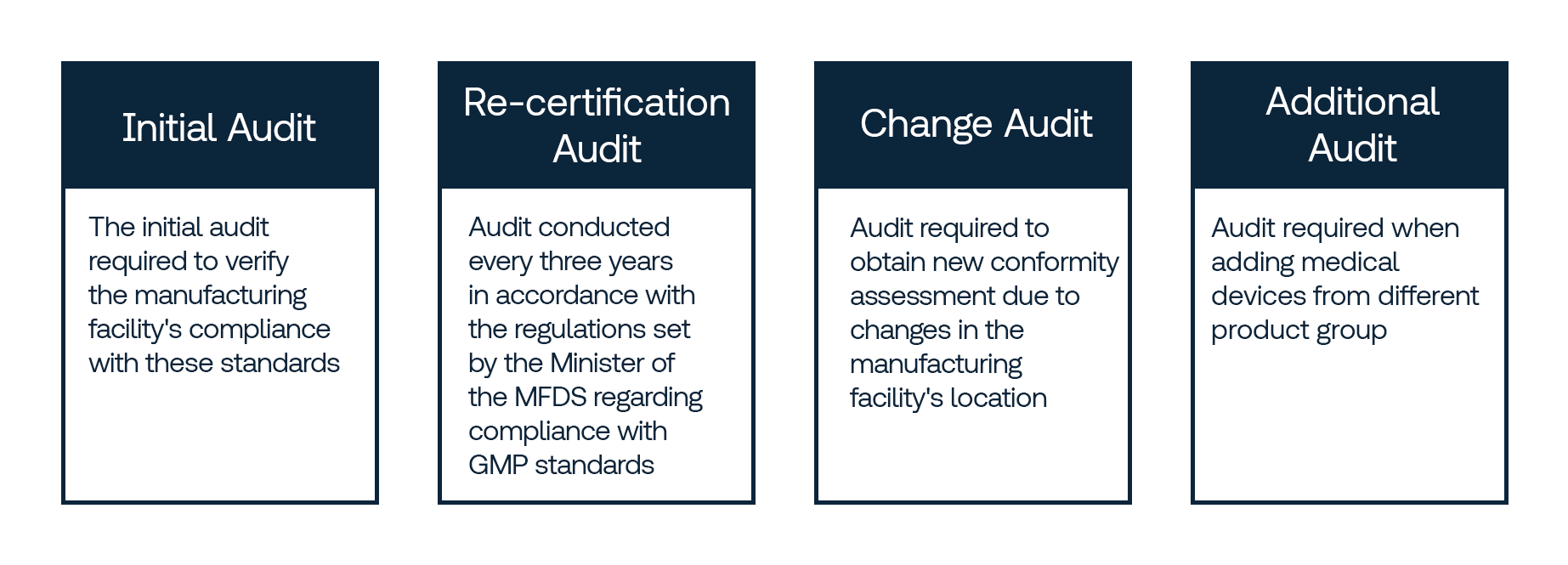
Services Offered
- Audit for assessment of medical devices for conformity with manufacturing and quality management standards
- Audit for assessment of in vitro diagnostic medical devices for conformity with manufacturing and quality management standards
- Issuance of certificate of GMP
Contact TÜV SÜD for Korea GMP audit
GMP Certification Procedure
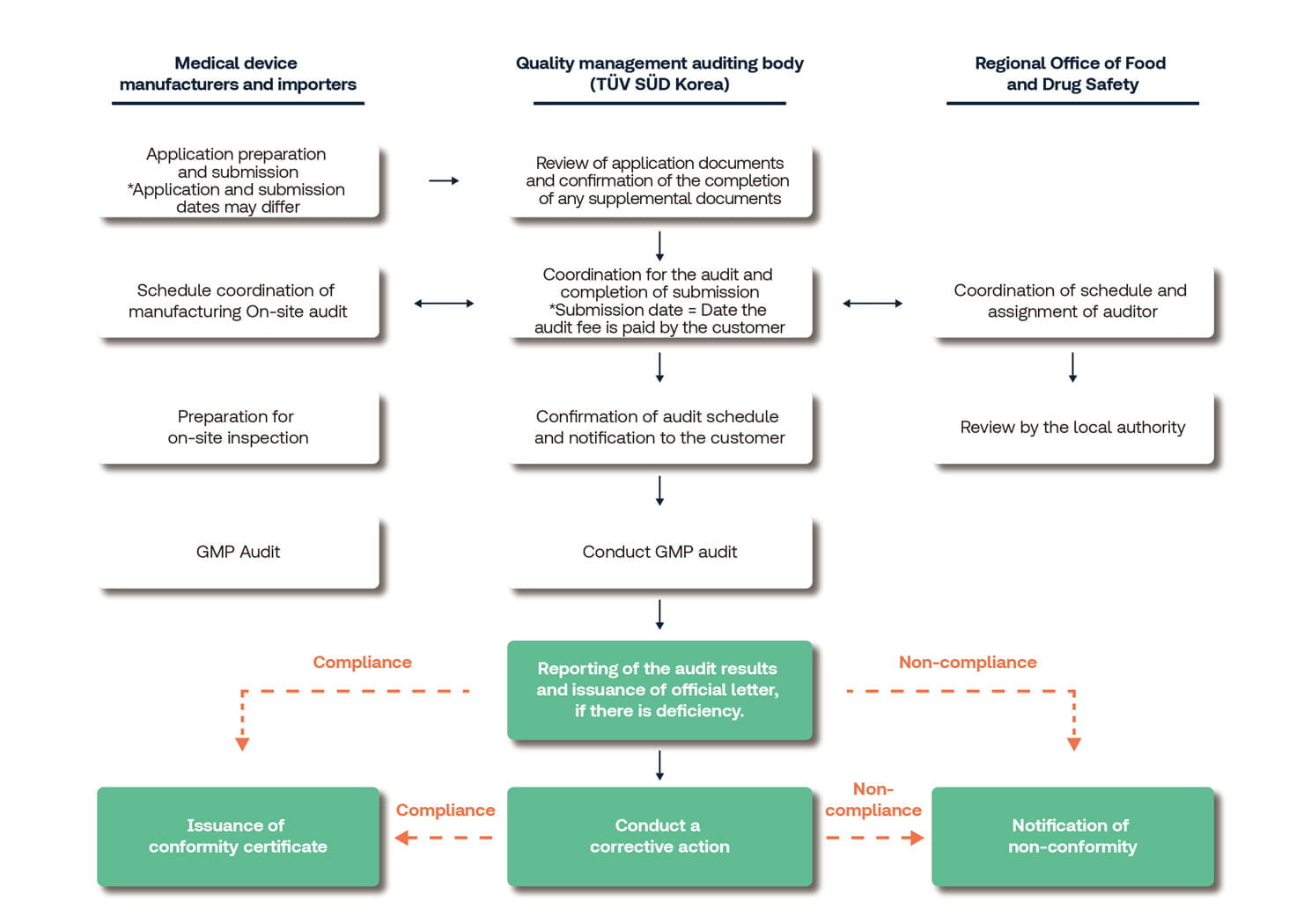
Application Procedure
- Complete the application according to the GMP Application Guideline.
- GMP application instructions for medical devices and in vitro diagnostic medical devices
- Download GMP application and forms for submission
- Submit via Integrated Information System for Medical Devices - Medical Device E-Service (allowing applicants to check their status in real-time using the E-Service)
HOW CAN TÜV SÜD SUPPORT YOU?
Since 1992, TÜV SÜD has been providing services in Korea through its branch offices in Seoul and Busan and testing facilities in Guro-gu, Seoul. Medical device experts at TÜV SÜD Korea have the extensive knowledge required for entering the South Korean market, as well as a thorough understanding and experience regarding relevant regulations. They facilitate efficient communication with regulatory authorities and local representatives of manufacturers in South Korea.
YOUR BENEFITS AT A GLANCE
- Support from publicly certified medical device experts - TÜV SÜD is one of the largest European certification body in the world. In collaboration with the Regulatory Foreign Affairs and Clinical Affairs Department, TÜV SÜD is recognised by major regulatory agencies worldwide, with extensive experience in all types of medical devices.
- Support from quality system certification and audit experts - Medical device approvals typically require the implementation of a quality management system. TÜV SÜD offers quality management system certification, and auditing and factory inspection services in accordance with most international standards and regulations. This enables customers to benefit from inspections and audits, while also saving time and costs.
- Active participation and engagement in the development of standards - The technical experts at TÜV SÜD actively engage in the development of standards related to medical devices and participate as members of major standard committees. Also, TÜV SÜD is a member of the European Association for Medical Devices of Notified Bodies (Team NB), where information on applicable standards and regulations for medical devices is exchanged and shared.
- One-stop solution for customers - TÜV SÜD offers testing services for major medical device markets in accordance with the international standards and regulations.
- Building collaborative relationships with medical device experts - TÜV SÜD has accumulated specialised knowledge in medical device-related technologies and regulations over a long period, making it a trusted partner for a range of entities, from global manufacturers to local research and development companies.
- Local expert support - Our local experts provide services in your language based on their rich knowledge and understanding of the respective region.
Contact TÜV SÜD for Korea GMP Audit



